Exogenus Therapeutics
Promoting development and commercialization of extracellular vesicles and other nanoparticles to tackle major healthcare challenges
Exogenus Therapeutics Chooses Lonza to Develop a GMP-Compliant Process to Manufacture Exo-101, its Lead Exosome-Based Candidate
Collaborative R&D
Unlock Innovation Through Collaborative R&D
Together, we can accelerate development, share expertise, and create the technologies of the future.
Pipeline
Explore the progress of our innovations, from discovery to clinical development
Our Team
Get to Know the Faces behind Exogenus Therapeutics
IP & Publications
Science First!
We believe that impactful discoveries should be open and transparent.
latest news
Exogenus Therapeutics is thrilled to announce the granting of a new patent in the United States, marking a major milestone in the company’s global expansion. This is the second region to recognize the patent family titled: “Compositions comprising small extracellular vesicles derived from umbilical cord blood mononuclear cells with anti-inflammatory and immunomodulatory properties and process for obtaining them.” The same patent family has already been granted in Japan. This innovative technology harnesses Extracellular Vesicles naturally released by umbilical cord blood cells, pre-conditioned in a specific environment, to deliver a novel therapeutic approach for regenerative medicine. Through extensive research, Exogenus has fully characterized these exosomes and their bioactivity, revealing a multifactorial mode of action characterized by an anti-inflammatory and immunomodulatory effect that sets the foundation for next-generation regenerative therapies. Exogenus has also evolved the methods to produce and purify the Extracellular Vesicles with these properties, and which can be utilized for production of Extracellular Vesicles-based products in GMP conditions. Joana Simões Correia, CEO and inventor of the patent, commented: “Securing this U.S. patent is a significant achievement for Exogenus Therapeutics. It strengthens our intellectual property portfolio, opens doors to a robust international market, and reinforces our commitment to pioneering innovation in...
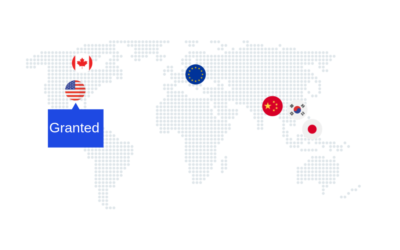
We are pleased to announce that Exogenus Therapeutics has been granted another patent, now in Europe! This is the fifth region to recognize the patent family titled “Use of umbilical cord blood-derived exosomes for tissue repair.” This patent family has also been granted in Canada, China, Japan and Korea. This invention leverages exosomes naturally released by umbilical cord blood cells, subjected to a specific pre-conditioning environment, and provides a therapeutic approach for regenerative medicine. Exogenus has thoroughly characterized these exosomes and their bioactivity, uncovering a multifactorial mechanism of action. Our CEO, Joana Correia, also inventor of the patent, stated: “This new European patent is a significant achievement for Exogenus Therapeutics. It opens doors to a robust international market, strengthens our patent portfolio, and highlights our commitment to innovation.” Exogenus Therapeutics' patent portfolio includes three families related to its lead product, Exo-101, covering different aspects of its composition, manufacturing process, and therapeutic applications (including inflammatory and fibrotic diseases). As a leading exosome biotech company, Exogenus is dedicated to developing innovative, safe, and effective therapies for patients with significant unmet needs. The company now plans to broaden the applications of its proprietary exosomes to treat a diverse range of conditions involving tissue...
Exogenus Therapeutics Chooses Lonza to Develop a GMP-Compliant Process to Manufacture Exo-101, its Lead Exosome-Based Candidate Lonza to develop a GMP-compliant process for Exo-101 manufacturing from its Siena (IT) site Exo-101 has shown safety and efficacy in multiple pre-clinical trials, and is expected to reach patients in 2027 Basel, Switzerland and Cantanhede, Portugal, 11 March 2025 – Exogenus Therapeutics, a Portuguese biotech company, and Lonza, a global development and manufacturing partner to the pharmaceutical and biotech markets, announced today a collaboration to develop Exo-101, Exogenus’ exosome-based lead candidate.Exogenus Therapeutics specializes in the development of healthcare solutions based on extracellular vesicles, including exosomes and other nanotechnologies. The company’s lead product, Exo-101, derived from immunologically privileged cells from umbilical cord blood, has been shown to have regenerative, anti‑inflammatory and immunomodulatory properties in multiple preclinical models and is expected to reach patients in 2027. Its multifactorial mode-of-action, mediated by a cocktail of small RNAs, proteins and anti-inflammatory lipids, provides the foundation for the drug’s therapeutic potential and positive safety profile[1],[2]. Exo-101 targets patients lacking effective treatment options primarily in the areas of tissue regeneration and inflammatory diseases.Under the agreement, Lonza will leverage its expertise in exosome development and analytical services from its Siena...